About Sanz
Who is Sanz.
Sanz – Your Trusted Partner in Aseptic Pharmaceutical Manufacturing
Sanz is a leading provider of advanced pharmaceutical machinery, specializing in the design, development, and manufacturing of high-performance washing, sterilizing, filling, and closing machines for vials, ampoules, syringes, and cartridges. With decades of industry expertise, we deliver cutting-edge solutions that operate as standalone units or seamlessly integrate into complete production lines—all under strict sterile conditions.
Why Choose Sanz

Experienced Team
- 30+ senior engineers with an average of 15 years in aseptic equipment R&D
- Masters both EU/US and China GMP requirements, offering full-spectrum support from mechanical design to process validation
- Ensures compliance-ready equipment that meets your production standards from day one
We’ve solved challenges you haven’t even encountered yet
Steadily Working Machine
- Core components sourced from Germany/Japan, triple-redundancy design for critical stations
- Proven through 10,000+ hours of continuous production with 60% lower failure rate than industry average
- Eliminate production disruptions caused by equipment downtime
Reliably stable machinery protects your productivity and maximizes RO


Customized Solutions
- Tailored machine configurations for your exact formulation characteristics – whether lyophilized powders, viscous biologics, or sensitive vaccines.
- Modular designs that adapt to your container mix (vials/ ampoules/ syringes), throughput requirements, and cleanroom constraints
- Every modification pre-validated to maintain full GMP compliance, with documentation packages for seamless regulatory approval.
Seek the right solution, not the biggest.
Lifecycle Service
- Global 12-hour rapid response network covering Asia/ Europe/ Americas
- Comprehensive IQ/OQ/PQ validation, operator training, and preventive maintenance programs
- Full access to OEM-certified spare parts with full traceability – ensuring identical performance to original equipment and maintaining GMP compliance.
Seek the right solution, not the biggest.

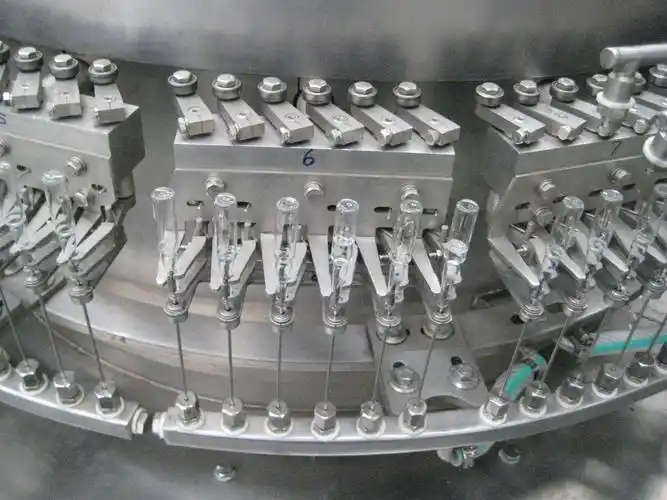
Project Cases





Challenge you May Face

The communication is hard, the supplier don’t understand what we want
They can’t provide full set of documents for validation
I don’t know if the machine design is comply with the GMP regulation until the machine production is finished, which is too late
I lost control of the production process
The deepen design is not standardized and hard to realize
It takes a lot of time finding the supplier who can supply both clean room system and pharmaceutical filling machines.
The spare parts are quite expensive.
- xxxxxxxxxxxxxxxxxxxxxxxxxxx
THAT’S WHY SANZ IS HERE!
